Exploring the Relationship Between Anger and Conditions like Anxiety and Depression Anger is a complex emotion that often signals more…


Exploring the Relationship Between Anger and Conditions like Anxiety and Depression Anger is a complex emotion that often signals more…
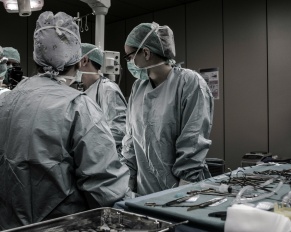
Corpectomy is a specialized surgical procedure recommended by spinal specialists for severe cases where the spinal cord or nerve roots…
Tooth trauma, an unexpected and often alarming event, can occur at any time due to various reasons such as sports…

In today’s fast-paced world, being prepared for unexpected health emergencies is more crucial than ever. While many of us have…
In the realm of healthcare, the regulation of medical devices in Canada stands as a critical framework designed to safeguard…

Temporomandibular Joint (TMJ) disorders represent a complex and often misunderstood condition that affects the jaw joint and the muscles responsible…
The pathway to securing FDA approval for a new drug is a testament to the blend of scientific rigor, innovation,…
The Food and Drug Administration (FDA) stands as a cornerstone of the United States healthcare system, tasked with the monumental…

In the complex and highly regulated world of healthcare, the introduction of the Electronic Common Technical Document (eCTD) has revolutionized…
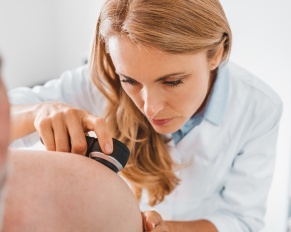
The sun’s golden rays, while a source of warmth and vitamin D, cast a long shadow over our skin’s health,…