Your teeth stay porous for about 48 hours after teeth whitening in New Jersey. That makes them more susceptible to…
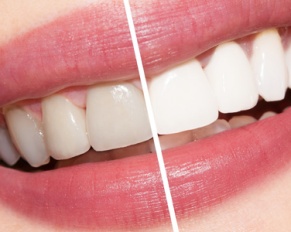

Your teeth stay porous for about 48 hours after teeth whitening in New Jersey. That makes them more susceptible to…
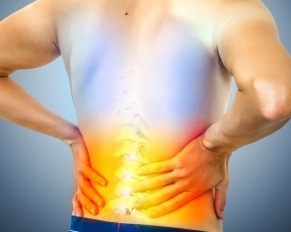
Pain in the lower left side of the back is common, frustrating, and can stem from numerous causes, including muscle…

Mold is an invisible threat that can quietly affect a senior’s health before anyone realizes what’s happening. For older adults—especially…

Lower back pain is one of the most common physical complaints during pregnancy. In fact, studies suggest 50% to 70%…

Restorative dentistry repairs or replaces teeth to restore function, appearance, and oral health. Usually, the affected teeth are damaged, decayed,…

Back pain is one of the most common complaints among adults over the age of 65. For many seniors, it…

Grief is a deeply personal journey—one that no one should have to walk alone. For seniors, the pain of loss…

Despite decades of awareness campaigns and warning labels, millions of people still smoke daily, and many underestimate just how deep…

Multilevel spondylosis is a spinal condition involving degenerative changes across many levels of the cervical or lumbar spine. It’s manageable…
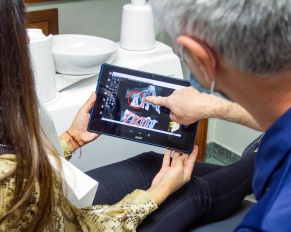
Millions of people get dental sedation treatments to ease their anxiety or pain every year. But, the medications involved with…